Real-World Bispecific Antibodies in Multiple Myeloma in the Netherlands (T-REX): Dosing Intervals and Infection Risk
Bispecific antibodies (BsAbs) are approved for treating relapsed/refractory multiple myeloma (RRMM) after ≥3 prior lines of therapy (LOT), if triple-class exposed (TCE). Real-world data are essential to evaluate efficacy and safety beyond clinical trials.
This nationwide, multicenter observational study collects real-world data on teclistamab (TEC; anti-BCMA) and talquetamab (TAL; anti-GPRC5D) for RRMM patients treated in a compassionate use program, focusing on infection occurrence, prophylactic measures, treatment schedule modifications, and quality of life (QoL) using EORTC QLQ-C30 and QLQ-MY20 questionnaires.
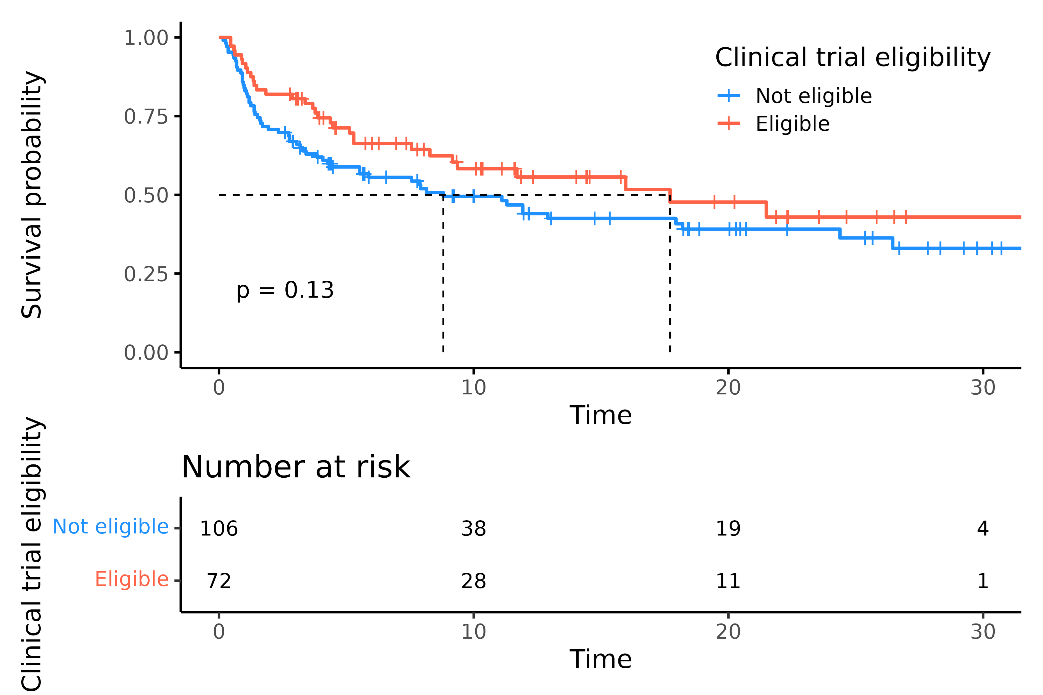
As of May 28, 2025, 337 RRMM patients received TEC and 39 received TAL in the Netherlands. Currently, 267 patients are included in the registry from 13 hospitals, with a median of 4 prior LOT; all were TCE, and 78% were triple-class refractory. Four patients had prior BCMA CAR-T therapy, and 30 received sequential TEC/TAL. 56% would not have met MajesTEC-1 or MonumenTAL-1 trial eligibility. Among 179 TEC-treated patients, the overall response rate (ORR) was 65%, with 56% achieving ≥VGPR at 9.2 months median follow-up (mFU). Median progression-free survival (mPFS) was 11.9 months; mPFS was 8.8 months in trial-ineligible patients vs. 17.7 months in eligible patients (attachment1). Median duration of response was not reached at 11.2 months, with 58% maintaining response at 24 months.CRS and ICANS occurred in 51% and 6% of patients, respectively, mostly grade 1–2, tocilizumab was administered to 41%. Infections were reported in 67% of patients (grade ≥3 in 32%); 73% received IVIG, 96% valaciclovir, and 92% cotrimoxazole.
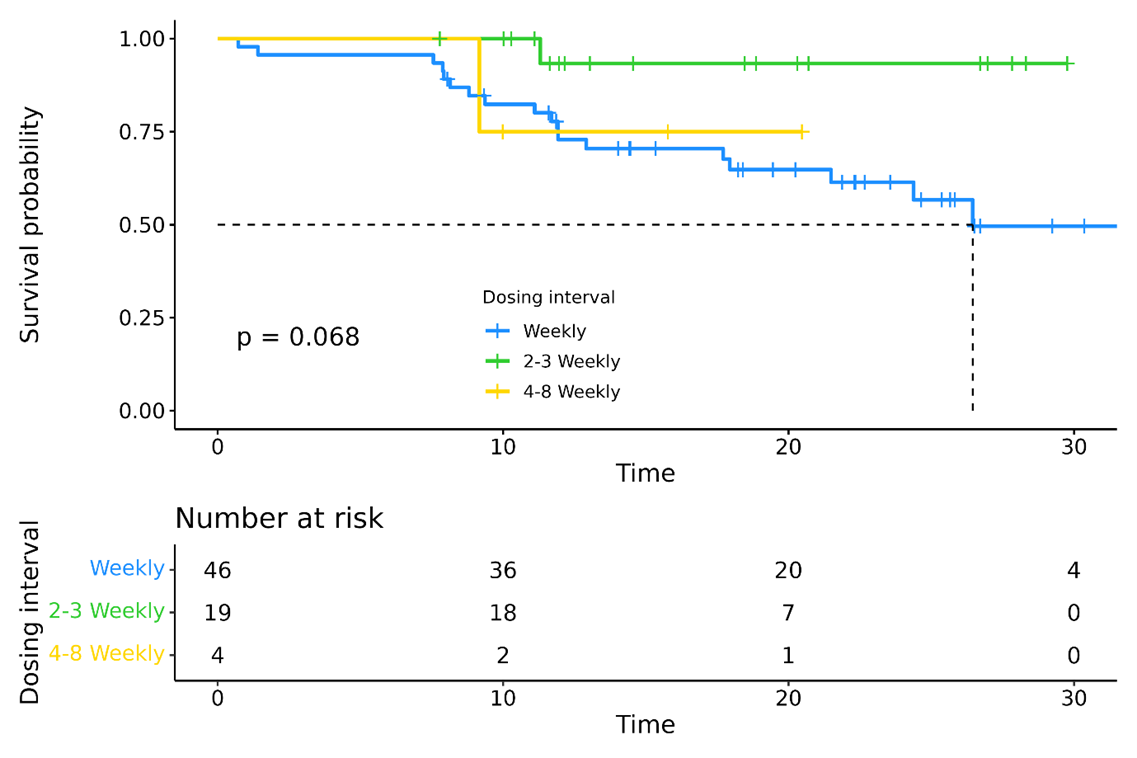
In TEC-treated patients, infection rates declined with longer dosing intervals: 38.1 per 100 patient-months during weekly dosing vs. 22.3 and 26.0 for 2–3-weekly and 4–8-weekly dosing, respectively (p < 0.005). To assess the effect of early interval extension, patients receiving weekly vs. prolonged dosing at month 4 were evaluated. Infection severity differed by interval: weekly (n=46) 15% infection-free, 31% grade ≥3; every 2-3 weeks (n=19) 17% infection-free, 17% grade ≥3; every 4–8 weeks (n=4) 75% grade 1, 25% grade 3. PFS was not significantly different between different dosing intervals (attachment2).
Among 25 TAL-treated patients, ORR was 68%, with 48% achieving ≥VGPR at 7.2 months mFU; mPFS was 5.8 months. Of these, 80% had previously received TEC; their ORR with TAL was 60%, with 45% achieving ≥VGPR at 7.3 months mFU.
Updated results will be presented at DHC.
Response rates and PFS align with clinical trial outcomes, confirming effectiveness in real-world settings, including patients who would not have met study criteria. Infectious complications were frequent, but hospitalization rates were lower, likely due to prophylactic measures and adjusted dosing. Ongoing QoL data collection will provide further insights into the impact of these therapies.