Treatment sequencing and outcomes beyond second-line treatment in patients with chronic lymphocytic leukemia: a population-based analysis
The therapeutic landscape of chronic lymphocytic leukemia (CLL) has evolved significantly, with both chemo-immunotherapy (CIT) and targeted therapies (TT). These modalities have markedly prolonged progression free survival (PFS) and the more clinically relevant parameter time to next treatment (TTNT), often extending over several years. However, long-term real-world data on treatment sequencing and outcomes beyond second-line therapy (2L) are lacking. To address this knowledge gap, we conducted a retrospective analysis of treatment patterns and outcomes in a real-world CLL cohort over a 20-year period.
In this retrospective, observational population-based study, we enrolled all patients newly diagnosed with CLL between January 2005 and January 2024, who received CIT or TT as first-line treatment (1L) at one of the four Frisian hospitals. Diagnosis and treatment were conducted in accordance with the International Workshop on Chronic Lymphocytic Leukemia (iwCLL) criteria [1]. For each patient, data were collected regarding biological disease characteristics, treatment regimens, and clinical outcomes. Clinical outcomes included TTNT and overall survival (OS). Data were analysed using SPSS Statistics 28, including survival analysis using Kaplan-Meier curves, the log-rank test and Cox regression.
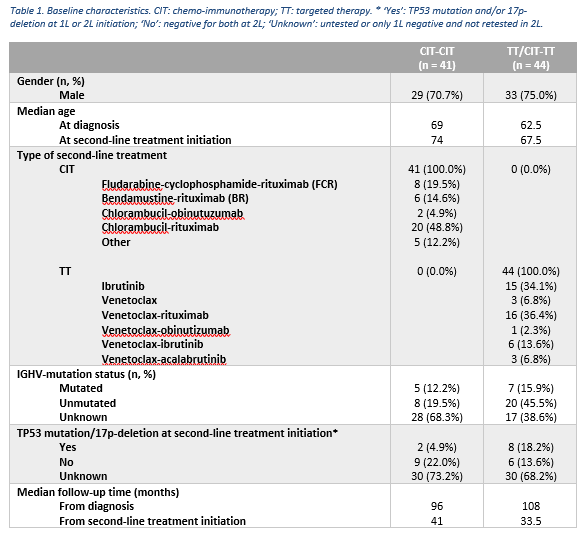
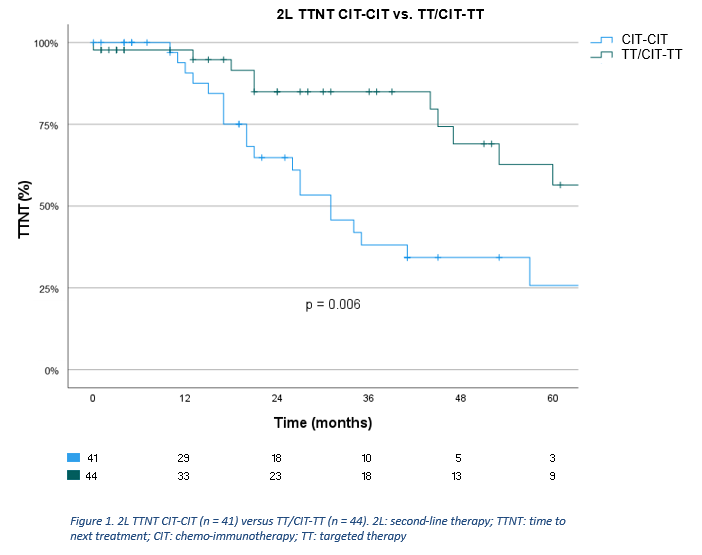
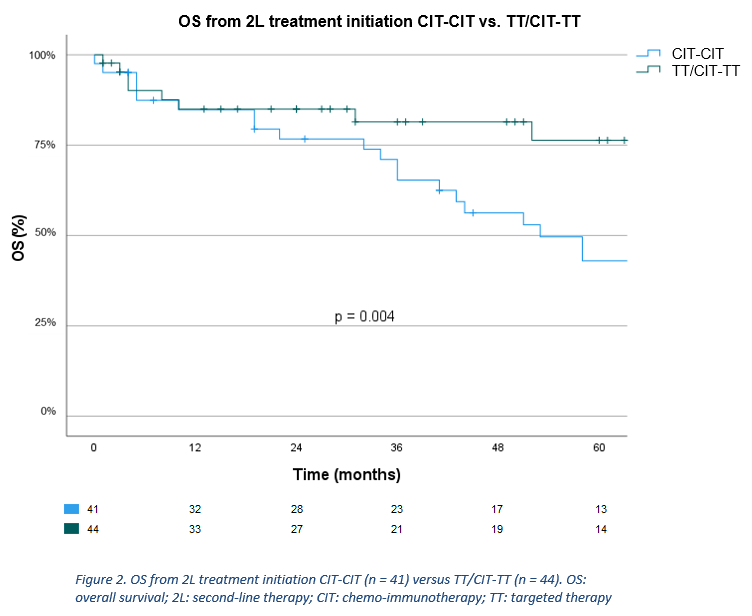
Of the 695 eligible patients, 282 (40.6%) received treatment, of whom 183 received CIT (64.9%), 33 TT (11.7%), 65 chemotherapy (23.0%), and 1 another treatment (0.4%) in 1L. Of 183 patients who received CIT in 1L, 41 received CIT (22.4%) in 2L (CIT-CIT). Of 216 patients who received CIT or TT in 1L, 44 received TT (20.4%) in 2L (TT/CIT-TT). Baseline characteristics of aforementioned subgroups are shown in table 1. Median follow-up time of the analyzed cohort was 104 months. Three-year 2L TTNT was 61.1% overall (median 53 months), and 38.1% (median 31 months) and 85.0% (median 77 months) for CIT-CIT and TT/CIT-TT, respectively. Univariate Cox regression showed patients receiving TT/CIT-TT had a significantly longer 2L TTNT compared to CIT-CIT (HR=0.39, 95%CI [0.19-0.78], p=0.008) (figure 1). Median OS from 2L was 78 months overall, while it was 53 months and not reached for CIT-CIT and TT/CIT-TT, respectively. Univariate Cox regression showed median OS from 2L was significantly longer for TT/CIT-TT compared to CIT-CIT (HR=0.35, 95%CI [0.16-0.74], p = 0.006) (figure 2). In the CIT-CIT subgroup, patients who received TT in later lines had improved OS from 2L compared to those who never received TT (HR=5.38, 95%CI [2.18-13.27], p<0.001).
This population-based analysis showed that in patients with CLL, targeted therapy administered in second-line was associated with a statistically significant longer TTNT and improved OS compared to chemo-immunotherapy. However, interpretation of these results is limited by missing data, particularly regarding mutational status at the start of 2L treatment.